Shimadzu is collaborating with the University Medical Center Göttingen (UMG), one of Germany’s leading university medical facilities. The collaboration will focus on the development of new clinical laboratory methods using liquid chromatography-mass spectrometry (LC-MS) for therapeutic drug monitoring (TDM) analysis.
TDM plays a vital role in providing precision medical care. TDM analysis measures the concentration of drugs currently in the blood to determine the required next dosage of a drug, e.g. antibiotics, antiepileptics, antidepressants etc. That means that it is especially important for a clinical laboratory to test a sample and return the result to the doctor as swiftly as possible. This is especially true for emergency patients who require special treatment. Because the standard dosage may not meet their needs in Intensive Care Unit, rapid test results are crucial for doctors to properly treat patients and save lives.
The conventional method for conducting TDM tests has been to use an immunoassay. More recently, however, clinical laboratories have been turning to LC-MS systems for this purpose. Among the advantages of LC-MS are greater accuracy and flexibility. Despite this overall superiority, methods using LC-MS have previously been difficult to apply in emergency cases because of the complexity of sample preparation. For instance, the flexibility of the LC-MS system also means that each target group of drugs requires its own column, mobile phase and analysis conditions such as temperature. This means that it can take time to switch methods to start a new sample analysis. And that can make it difficult for a clinical laboratory to quickly return analysis results to the doctors who need them.
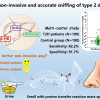
However, Shimadzu’s CLAM-2030 has shown great potential in simplifying, streamlining and speeding up LC-MS testing. CLAM-2030 is a fully automated sample preparation module for LC-MS. Lab technicians simply place blood collection tubes in position and the module automatically performs sample pretreatment steps up to LC/MS/MS analysis.
Further developing and testing this potential will form the core of the new collaboration between Shimadzu and UMG. Working together with Professor Andreas Fischer, Dr Frank Streit and other leading experts at UMG, new methods for multiple target groups of drugs will be developed. The team will also use CLAM-2030 to optimise these new methods so that clinical laboratories can switch methods easily and flexibly—and most importantly quickly. This will mean that the more accurate results of LC-MS testing can be used by doctors more rapidly than ever before.